TENOFOVIR
Specifications
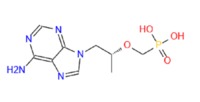
TENOFOVIR
C9H14N5O4P
147127-20-6
Tenofovir disoproxil (Viread) is the first nucleotide analogue approved by the American FDA to treat HIV-1 infections. Tenofovir disoproxil is a drug used in the AIDS cocktail treatment method, and research shows that it has the ability to increase monkeys’ immunity to immunodeficiency viruses (similar to the human AIDS virus). Tenofovir disoproxil is used in combination with other reverse transcriptase inhibitors to treat HIV-1 infections and hepatitis B.
Tenofovir disoproxil is an acyclic nucleoside antivirus drug and has an inhibiting effect on HBV multi-enzyme complexes and HIV reverse transcriptase. Its active content tenofovir phosphonate directly competitively binds to natural deoxyribose substrate to inhibit the virus multi-enzyme complex and inserts itself into the DNA to end the nucleotide chain. Tenofovir disoproxil is barely absorbed by the gastrointestinal duct, so it undergoes esterification and ionization to become tenofovir ester fumarate. Tenofovir is soluble in water and can be quickly absorbed and decomposed into the active substance tenofovir, which then transforms into the active metabolite tenofovir phosphonate. As this drug is not metabolized by the CYP450 enzyme system, it has a very low chance of drug interactions caused by this enzyme.
Tenofovir disoproxil reaches peak blood concentration 1-2 hours after intake. Tenofovir disoproxil’s bioavailability increases by about 40% when taken with food. The intracellular half-life of tenofovir phosphonate is about 10 hours, so doses can be taken once daily. This drug is mainly filtered through renal glomeruli and excreted through the renal tubule transport system, with 70-80% excreted in its original form through urine.
- Country: China (Mainland)
- Business Type: Hangzhou Huisheng Biotech Pharmaceutical Co.,Ltd
- Market: The Middle East,Europea,Russia,and South America and US market
- Founded Year: 2002
- Contact: Tibi Teng