FEBUXOSTAT
Specifications
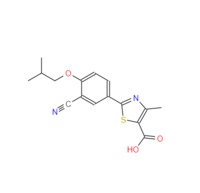
| Febuxostat, a selective xanthine oxidase inhibitor, was launched for the chronic management of hyperuricemia in patients with gout. Hyperuricemia is defined as a serum uric acid concentration exceeding the limit of solubility. It predisposes affected persons to gout, a disease characterized by the formation of crystals of monosodium urate or uric acid from supersaturated fluids in joints and other tissues. Crystal deposition is asymptomatic, but it is revealed by bouts of joint inflammation. If left untreated, further crystals accumulate in joints and can form deposits known as tophi. A major aim in gout management is the long-term reduction of serum uric acid concentrations below saturation levels, as this results in crystal dissolution and eventual disappearance. Febuxostat is a nonpurine derivative with higher potency and selectivity than allopurinol for inhibiting xanthine oxidase. It completely inhibits human xanthine oxidase activity in the lung cancer cell line A549, whereas the activities of other enzymes involved in purine or pyrimidine metabolism (e.g., purine nucleoside phosphorylase, adenosine deaminase, and pyrimidine nucleoside phosphorylase) are affected by <4%. The incidence of adverse events such as dizziness, diarrhea, headache, and nausea with febuxostat was similar to allopurinol. Febuxostat is contraindicated in patients being treated with the xanthine oxidase substrates such as azathioprine, mercaptopurine, and theophylline. Febuxostat can be synthesized in a multistep sequence from 2,4-dicyanophenol, starting with the alkylation of the phenolic hydroxyl group with isobutyl bromide and potassium carbonate, followed by treatment with thioacetamide in hot dimethyl formamide to yield 3-cyano-4-isobutoxythiobenzamide. Cyclization of the thioamide group with 2-chloroacetoacetic acid ethyl ester in refluxing ethanol affords 2-(3-cyano-4-isoutoxyphenyl)-4-methylthiazole-5-carboxylic acid ethyl ester, which is hydrolyzed with sodium hydroxide to produce febuxostat. |
- Country: China (Mainland)
- Business Type: Hangzhou Huisheng Biotech Pharmaceutical Co.,Ltd
- Market: The Middle East,Europea,Russia,and South America and US market
- Founded Year: 2002
- Contact: Tibi Teng