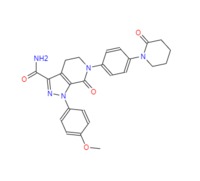
Apixaban
Product Quick Detail
- Minimum Order
- 1
- Packaging
- 25kg/drum
- Delivery
- 15 Days
Specifications
Eliquis (apixaban), a direct inhibitor of factor Xa (FXa), was approved by the European Commission on May 18, 2011 for prevention of venous thromboembolic events (VTE) in adult patients who
have undergone elective hip or knee replacement surgery. The discovery of apixaban was the culmination of a succession of novel and innovative medicinal chemistry discoveries starting with the
identification of nonpeptide leads, rational drug design using computer-aided and X-ray crystallographic information, and the building of drug-like properties through the systematic replacement of
basic groups with neutral moieties. Apixaban arose from modifications to razaxaban by constraining a pyrazole amide to form a bicyclic pyrazolo-pyridinone scaffold. Optimization of the P1 group
resulted in the identification of the nonbasic methoxy phenyl group, while a P4 piperidinone improved the balance of potency and pharmacokinetics with low Vdss. The synthesis of apixaban begins with
the generation of a hydrazone of 4-methoxyaniline which is then used in a 3+2 cycloaddition with a dihydropiperidinone to form a bicyclic pyrazolo-pyridinone scaffold. The distal piperidinone group
is installed using an Ullmann coupling reaction followed by aminolysis of an ethyl ester on the pyrazole ring to complete the synthesis of apixaban.
- Country: China (Mainland)
- Business Type: Hangzhou Huisheng Biotech Pharmaceutical Co.,Ltd
- Market:The Middle East,Europea,Russia,and South America and US market
- Founded Year:2002
- Address:
- Contact:Tibi Teng
Other products from HangZhou HuiSheng Biotech Pharmaceutical Co., Ltd
Relate products of Apixaban
Apixaban
慧生药业位于杭州市,经过15年的努力已发展成为领先的制药公司。 我们产品库中的数百种 API 均来自 GMP 来源并具有 DMF/CEP 证书,质量上乘。 ...
Eliquis Apixaban 5 mg 20 tabs
Eliquis (apixaban) helps to prevent platelets in your blood from sticking together and forming a blood clot. Eliquis is used to lower the risk of stroke caused by a blood clot in people with a heart rhythm disorder called Atrial fibrillation. Boykaiori18@gmail.com ...