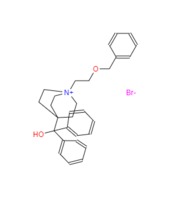
Umeclidinium bromide
Specifications
| Commercially available ethyl isonipecotate (278) was alkylated with 1-bromo-2-chloroethane in the presence of K2CO3 in acetone to give ethyl 1-(2-chloroethyl)piperidine-4-carboxylate (279). This material was then treated with lithium diisopropylamine (LDA) in THF to affect a transannular substitution reaction resulting in the cyclized quinuclidine 280 in 96% yield. Excess of phenyllithium was added to ester 280 in THF starting at low temperature then gradually warming to room temperature to give tertiary alcohol 281 in 61% yield. Amine 281 was finally alkylated with benzyl 2- bromoethyl ether (282) in MeCN/CHCl3 at elevated temperatures to afford umeclidinium bromide (XXXV) in 69% yield. |
- Country: China (Mainland)
- Business Type: Hangzhou Huisheng Biotech Pharmaceutical Co.,Ltd
- Market: The Middle East,Europea,Russia,and South America and US market
- Founded Year: 2002
- Contact: Tibi Teng