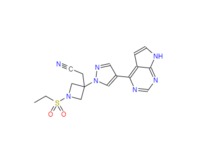
Baricitinib
Specifications
The oral, targeted synthetic DMARD (tsDMARD)[2], JAK1 and JAK2 inhibitor baricitinib (Olumiant) is a novel small molecule[1] that is approved in the EU[3] and Japan[4] for the treatment of adults with rheumatoid arthritis RA (moderate or severe active[3]), who responded inadequately to (other treatments[4]) or who were intolerant of C1 DMARD[3]. JAK is a tyrosine kinase that plays crucial roles[5] in cytokine receptor binding-triggered signal transduction activating the transcription factor signal transducers and activator of transcription (STAT). Activation of the receptor-bound JAKs is critical for initiating phosphorylation of the cytokine receptor and subsequent recruitment of one or more STATs. The phosphorylated STAT dimer is then actively and directly transported to the nucleus without involvement of any other kinases[6]. The JAK family consists of four members: JAK1, JAK2, JAK3, and TYK2. More than 40 different cytokines and growth factors have been shown to activate specific combination of JAKs and STATs. Genetic knockout studies have shown that JAKs and STATs have highly specific functions in the control of various immune responses. Baricitinib represents a selective inhibitor of JAKs with excellent potency and selectivity for JAK2 (IC50=5.7nM) and JAK1 (IC50=5.9nM), and less potency and selectivity for JAK3 (IC50 >400nM), or TYK2 (IC50=53nM)[7]. Although baricitinib potently inhibits JAKs, it does not significantly inhibit (<30% inhibition) a broad panel of 26 other kinases when tested at 200nM[7].
- Country: China (Mainland)
- Business Type: Hangzhou Huisheng Biotech Pharmaceutical Co.,Ltd
- Market: The Middle East,Europea,Russia,and South America and US market
- Founded Year: 2002
- Contact: Tibi Teng