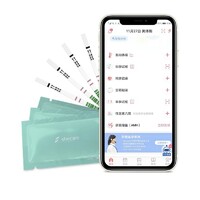
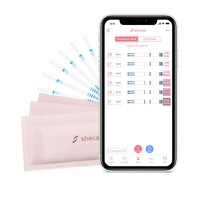
Shecare COVID-19 IgG/IgM Test Kit
Specifications
COVID-19 IgG/IgM Test Kit
COVID 19 test kit (Whole Blood/Serum/Plasma) is a solid phase immunochromatographic assay for the rapid, qualitative and differential detection of IgG and IgM antibodies to 2019 Novel Coronavirusi
in human whole blood, serum or plasma.
The covid 19 home test kit is a lateral flow immunochromatographic assay. The test uses anti-human IgM antibody (test line IgM) , anti-human IgG (test line IgG) and goat anti-rabbit IgG (control
line C) immobilised on a nitrocellulose strip. The burgundy colored conjugate pad contains colloidal gold conjugated to recombinant COVID-19 antigens conjugated with colloid gold (COVID-19
conjugates) and rabbit IgG-gold conjugates. When a specimen followed by assay buffer is added to the sample well, IgM &/or IgG antibodies if present, will bind to COVID-19 conjugates making
antigen antibodies complex. This complex migrates through nitrocellulose membrane by capillary action. When the complex meets the line of the corresponding immobilized antibody (anti-human IgM
&/or anit-human IgG) the complex is trapped forming a burgundy colored band which confirm a reactive test result.
Features of shecare home covid 19 test kit
Detects Presence of IgG/IgM in human fingerstick blood
COVID-19 igm and igg test kit (Whole Blood/Serum/Plasma) can be performed using either whole blood, serum or plasma.
The covid 19 test kit is suitable for clinical diagnosis of New Coronavirus infection. It is fast, simple, accurate and highly sensitive. It is widely used in clinical system, health care
institutions and scientific research.
The covid igm igg antibody test kit can be stored at room temperature or refrigerated (2-30°C). The test device is stable through the expiration date printed on the sealed pouch. The test device
must remain in the sealed pouch until use. DO NOT FREEZE. Do not use beyond the expiration date.
How to Use COVID-19 igg igm rapid test Kit
Test Procedure
Allow test kit, specimen, bufferand/or controls to equilibrate to room temperature(15-30°C) priorto testing.
1. Remove the test strip/cassette from the sealed foil pouch and use it as soon as possible. Best results will be obtained if the assay is performed within one hour.
2. Place the test device on a clean and level surface.
3. Add 4uL of whole blood to the sample pad(purple place with Colloidal gold) of the test strip,then add 2 drops (about 60 μL) of sample buffer to the buffer pad (top of the strip) immediately.
4. Wait for the colored line(s) to appear. The result should be read at 10 minutes. Positive results may be visible as soon as 2 minutes. Do not interpret the result after 15 minutes.
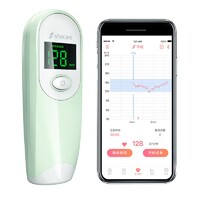
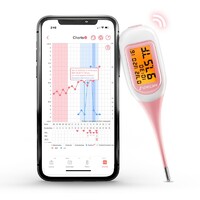
For more information about opk ovulation predictor kit and digital forehead thermometer, please feel free to contact us!
- Country: China (Mainland)
- Business Type: Manufacturer
- Market: Americas,Oceania
- Founded Year: 2013
- Address: Room 1206, Block B, Xueyan Building, Shuangqing Road, Haidian District, Beijing,China
- Contact: She care